
세계적 블록버스터 신약의 물질특허에 대한 Exclusive License 및 추가 R&D Collaboration Agreement 중에서 특허관련 계약조항을 인용하고 간략하게 살펴보겠습니다. 신약물질을 개발한 일본 제약회사 Shionogi에서 신약물질 특허권을 보유하고, 대형 제약회사 AstraZeneca에 대해 해당 특허의 전용실시권 허여 및 상업화 권리를 부여하는 기술이전 license 계약입니다. 이미 상당한 시간이 지난 오래 된 계약서이지만, 실제 엄청난 성공을 거둔 신약개발 기술이전 프로젝트로서. 실무자가 참고자료로 살펴볼 가치가 높다 생각합니다.
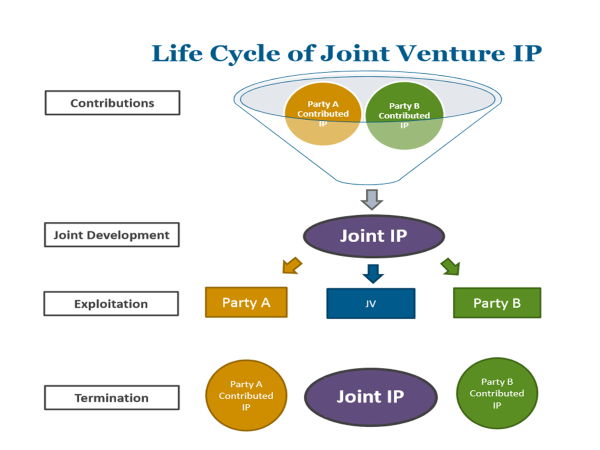
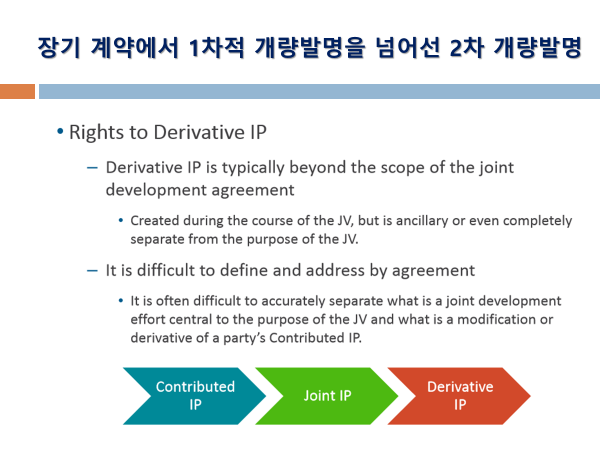
Article 7. Inventions (추가 연구개발 발명에 대한 권리관계 규정. 단독 발명의 경우 개발회사에 권리귀속 확인 + 공동발명은 공동으로 결정 권한 보유 및 비용 등 책임도 분담 규정)
Inventions which are made and which relate to the COMPOUNDS and/or LICENSED PRODUCTS shall belong to the PARTY making such invention. Each PARTY shall have the right to file, prosecute and maintain patent applications and patents covering inventions made solely by that PARTY.
If an invention is made jointly by the PARTIES, such invention shall be jointly owned. Neither PARTY shall file any patent application(s) containing such joint invention and/or any information or data received from the other PARTY without the prior written consent of the PARTY providing the information or data.
SHIONOGI and ZENECA shall mutually determine whether or not patent applications should be filed concerning such joint inventions, which PARTY shall be responsible for filing and prosecuting any patent applications filed, and share the costs in filing any patent applications, obtaining and maintaining any patents covering joint inventions.
Article 9. Representation and Warranty (진술 및 보증조항. 특허유효 및 권리 소유관계, 타인의 권리침해 여부 등에 관한 성실한 조사 및 그 결과에 따른 인식범위로 보증책임 제한. 무제한 보증 아님.)
| 9.1 | SHIONOGI represents and warrants that it is the owner of the entire right, title and interest in the PATENTS listed in Appendix II hereto and KNOW-HOW, and is entitled to grant the licenses specified herein. SHIONOGI further hereby represents and warrants that, to the best of its knowledge, the PATENTS owned or controlled by SHIONOGI or SHIONOGI’s AFFILIATES are being procured from the respective Patent Offices in accordance with all applicable laws and regulations. |
| 9.2 | SHIONOGI represents and warrants that it has full corporate power to enter into this Agreement and to carry out the provisions hereof. |
| 9.3 | ZENECA represents and warrants that it has full corporate power to enter into this Agreement and to carry out the provisions hereof. |
| 9.4 | SHIONOGI represents that, as of the EFFECTIVE DATE, it is not aware of any infringement or threatened infringement of the PATENTS or KNOW-HOW by a THIRD PARTY. |
| 9.5 | SHIONOGI represents and warrants that, to the best of SHIONOGI’s knowledge, ZENECA’s exploitation or use of the PATENTS and/or KNOW-HOW hereunder will not infringe any patent or other intellectual property right enjoyed by any THIRD PARTY (with the exception of Hoechst European Patent Applications No. 88120057.0 and any foreign applications or patents corresponding thereto). |
| 9.6 | SHIONOGI represents that, as of the EFFECTIVE DATE, SHIONOGI has no knowledge from which it can reasonably be inferred that the PATENTS are invalid. |
Article 11. PATENTS (특허출원, 심사 및 등록과장에서 라이센시의 검토 및 참여권리 보장. 특허권리 확보에 라이센시 참여권 부여)
| 11.1 | SHIONOGI has the responsibility to file, prosecute and maintain the PATENTS and shall bear all cost for the PATENTS, including fees and expenses paid to outside legal counsel and experts, direct costs of in-house counsel and filing, prosecution and maintenance expenses associated therewith. |
| 11.2 | SHIONOGI shall provide ZENECA with an opportunity to review and comment on the nature and text of new or pending applications for the PATENTS. |
| 11.3 | SHIONOGI shall advise ZENECA on an annual basis of progress in the prosecution of all patent applications and in the maintenance or extension of patents falling within the PATENTS and shall, on request, furnish ZENECA with a copy of the patent application, patent or other document pertinent to prosecution, maintenance or extension of such applications and patents. |
| 11.4 | No significant steps regarding prosecution of the PATENTS will be taken by SHIONOGI without prior consultation with ZENECA. In particular, no steps concerning European Patent Application No. 92111090.4 or US Patent 5,260,440 will be taken by SHIONOGI without prior consultation with ZENECA. |
| 11.5 | If SHIONOGI elects not to continue to prosecute a patent application or not to maintain or extend any patent application or patent within the PATENTS, SHIONOGI shall notify ZENECA not less than two (2) months before any relevant deadlines. Thereafter ZENECA shall have the right to pursue at its expense, and at its sole discretion, the prosecution, extension or maintenance of such application or patent. Any costs incurred by ZENECA pursuant to this shall be offset against royalties payable under Article 4. |
| 11.6 | ZENECA may request SHIONOGI to seek additional patent protection for the COMPOUNDS or LICENSED PRODUCTS in the TERRITORY, for example, by way of patent registration, patent of importation or revalidation, or the like. If SHIONOGI chooses to seek such additional patent protection, it shall do so at its own cost and in its own name. If SHIONOGI chooses not to seek such additional patent protection, ZENECA may require SHIONOGI to do so; provided ZENECA reimburses SHIONOGI for any reasonable expenses incurred in doing so. Such patent property shall then be included within the definition of PATENTS. |
| 11.7 | SHIONOGI shall immediately advise ZENECA of any certification filed under the U.S. “Drug Price Competition and Patent Term Restoration Act of 1984” (“ANDA ACT”) claiming that any PATENTS are invalid or claiming that the PATENTS will not be infringed by the manufacture, use or sale of a product for which an application under ANDA ACT is filed. |
| 11.8 | The PARTIES will cooperate with each other in gaining patent term extension(s) or the like, where applicable to the PATENTS in the TERRITORY, for example, under the U.S. “Drug Price Competition and Patent Term Restoration Act of 1984” or under a supplementary protection certificate in European countries. |
| 11.10 | Upon reasonable request of ZENECA, SHIONOGI will provide ZENECA with all necessary assistance relating to the PATENTS, including allowing ZENECA access to SHIONOGI’s files and documents and access to SHIONOGI’s personnel who may have possession of relevant information. |
Article 12. Infringement of PATENTS (라이선스 대상 특허권을 제3자가 침해할 경우 양당사자의 침해대응 책임 및 협력 방안, 대상 특허실시로 타인 권리를 침해하는 경우 분쟁 대응 책임 및 협력 방안 규정)
| 12.1 | In the event that ZENECA or SHIONOGI supposes that a THIRD PARTY may be infringing any of the PATENTS by the manufacture, use, distribution, marketing or sale of the COMPOUNDS and/or LICENSED PRODUCTS, ZENECA or SHIONOGI shall promptly notify the other PARTY in writing, identifying the infringer and the infringement complained of and furnishing the information upon which such determination is based. ZENECA shall be entitled, in its sole discretion but after notifying SHIONOGI, to take any measures deemed appropriate to stop such infringing activities by such THIRD PARTY in the TERRITORY or to grant to the infringing THIRD PARTY adequate rights and licenses necessary for continuing such activities in the TERRITORY so long as ZENECA remains in compliance with Article 4. Upon reasonable request by ZENECA and at ZENECA’s cost, SHIONOGI shall give ZENECA all reasonable information and assistance including allowing ZENECA access to SHIONOGI’s files and documents and access to SHIONOGI’s personnel who may have possession of relevant information, and if necessary to prosecute any legal action, joining in the legal action as a party. |
| 12.2 | ZENECA shall bear the cost of any action or measures taken in accordance with Article 12.1 and shall be entitled to receive any damages or remuneration received as a result of such action or measures. |
| 12.3 | In the event ZENECA decides, within sixty (60) days of becoming aware of an infringement, in its sole discretion, not to take any action against a THIRD PARTY deemed to infringe the PATENTS, ZENECA shall inform SHIONOGI in writing and SHIONOGI thereafter shall be entitled to pursue an action to stop such infringement in its own name and for its own account. Upon reasonable request by SHIONOGI and at SHIONOGI’s cost, ZENECA shall give SHIONOGI all reasonable information and assistance. Any damages or remuneration received as a result of such action shall be received by SHIONOGI. |
| 12.4 | In the event of any actual or threatened suit against ZENECA or its AFFILIATES, SUBLICENSEES or customers alleging that the exploitation or use of the PATENTS and/or KNOW-HOW hereunder infringes the patent or other intellectual property rights of a THIRD PARTY, ZENECA shall promptly give written notice to SHIONOGI. SHIONOGI will provide to ZENECA all reasonable assistance requested by ZENECA to defend or settle such suit and in particular SHIONOGI will promptly make available to ZENECA, free of charge, all information in its possession or control which will assist ZENECA in defending or otherwise dealing with such suit. ZENECA shall have the right to defend in its sole discretion such suit but shall consult with SHIONOGI before settling such suit. ZENECA shall not settle the suit without obtaining prior written consent of SHIONOGI which consent shall not be unreasonably withheld. If damages or costs are awarded against ZENECA for such infringement, or if the outcome of the suit is that ZENECA is ordered to or agrees to make payments or pay royalties to a THIRD PARTY in order to secure the right to continue the exploitation or use of the PATENTS and/or KNOW-HOW hereunder, then the following percentages of such damages, payments, or royalties shall be offset against royalties payable by ZENECA under Article 4: [***]. Notwithstanding the foregoing, in any event described above, SHIONOGI shall be entitled to receive at least [***] percent ([***]%) of the royalties due under Article 4 hereof from ZENECA in any one calendar year. |
[질문 또는 상담신청 입력하기]













 KASAN_항암제 신약물질 Lead Compound 발굴 공동연구개발 계약서 - 미국 벤처회사와 대기업 파트너 AstraZeneca 사이 기술이전 라이선스 영문계약서 [자문작성신속저비용].pdf
KASAN_항암제 신약물질 Lead Compound 발굴 공동연구개발 계약서 - 미국 벤처회사와 대기업 파트너 AstraZeneca 사이 기술이전 라이선스 영문계약서 [자문작성신속저비용].pdf














































 Astex_AZ_1심 판결.docx
Astex_AZ_1심 판결.docx





















